SIX
Strong Interactions
Uranium? . . .
Beyond the anecdotes of a film, what were they actually doing at Via Panisperna? Uranium does come into play, but not until much later, around the same time that Ettore’s mind collapsed, some five years before he finally abandoned the world. We can’t be sure that the prospect of nuclear weapons was central to what happened, but it certainly added complexity to his drama. Yet in this early period the Boys’ tale was still innocent—their work was almost philosophical in nature.
They toiled on the recently discovered theory of quantum mechanics, the construction ruling the behavior—or misbehavior—of the microworld, the home of the neutrino and other elementary particles. Quantum mechanics has a reputation for being bizarre: It affirms that the microscopic universe works in a radically new way—like the insane, the schizophrenic, the bipolar. No wonder that old school physicists, including Einstein, found themselves in a state of denial with regard to the new theory.
We can predict with pinpoint accuracy the future locations of large objects, such as planets and spacecraft, but it turns out that for electrons and nuclei we have to content ourselves with probabilities. They’re plagued by indeterminacy and are free to do as they wish, limiting the rein of the scientist. If humans can be fickle, electrons and nuclei are even worse. Imagine that an unstable isotope wakes up in an irascible mood. Will it decay or not? We can only predict probabilities for what is intrinsically an erratic, “irrational” process.
Indeterminacy permeates the microcosmos, and it’s even more insidious than day-to-day uncertainty. No one would flinch at the idea of asking where a car is and what its speed might be; ask any policeman giving you a speeding ticket. But a quantum particle would offer a dilemma: You can measure either its speed or its position exactly, but not both. The more you know about its position, the less you know about its speed and vice versa. You can also settle on a compromise between knowing its approximate speed and position, but indeterminacy can’t be avoided altogether. If a policeman told a quantum particle its location when it was allegedly found speeding, the particle could take the lying bastard to quantum court and win the case. This is essentially the content of the famous Heisenberg uncertainty principle.
Several aspects of quantum physics offer similar dilemmas. A quantum particle can sometimes behave like a wave—its “wave function” is a vibration spreading throughout space; but at other times it can behave like a lumpy object well localized in a particular place. Arthur Eddington descriptively dubbed quantum objects wavicles. To make it more schizophrenic, whether a wavicle acts like a lumpy particle or an extended wave depends on how physicists ask questions about it. Quantum mechanics is like the fraudulent statistics supplied by politicians—who phrase the questions to elicit the answers they want to hear. In quantum theory the question affects the answer.
If all of this weren’t enough to make Sir Isaac Newton barf in his tomb, quantum mechanics then allows superpositions of opposites. Infamously, Schrödinger’s cat can be 20 percent dead and 80 percent alive. The reluctant beast is placed in a cage where there’s a cyanide device attached to a quantum system (e.g., the radioactive decay of a single nucleus of an isotope). Quantum systems can be in superpositions of opposites: If I say that a nucleus has 20 percent probability of having decayed and an 80 percent probability of not having decayed, I mean that until I make an observation (ruled by those odds), the nucleus is in a ghostly superposition of the two. Therefore so is the cat, whose fate is correlated with the quantum process: She collapses into one “reality” only when an observation is made, say, by presenting her with a fish and noting enthusiasm or lack thereof.
Schrödinger’s cat is a contentious animal, created for shock value, but quantum mechanical superpositions in the microworld are entirely uncontroversial. They were discovered by means of “spin,” a major player in Majorana’s saga. Protons and electrons spin like tops, a property discovered in 1925 by two young Dutchmen, George Uhlenbeck and Samuel Goudsmit. But these spinning “tops” are unlike any other: Given a chosen direction for their rotation axis, their rotation “speed” is fixed, the only choice being whether they rotate one way or the other: “spin up” or “spin down” (see Figure 6.1). So important is spin to quantum mechanics that its central number, Planck’s constant (which plays a role in quantum physics akin to that of the speed of light in relativity), is a measure of “spinning.” In units of Planck’s constant, the spin of the electron and proton is half: either plus or minus half, depending on whether it is spinning up or down.
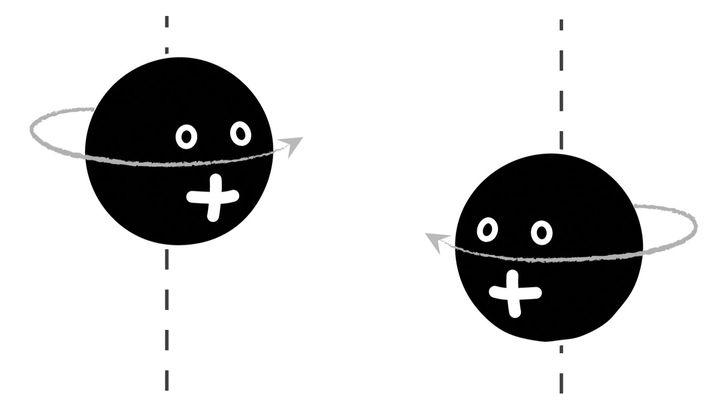
Figure 6.1: The spin of quantum particles, illustrated with a proton. Given a direction for the rotation axis (set, say, by an external magnetic field, here represented as a dashed line) the proton rotation “speed” is fixed, the only choice being whether it rotates one way or the other. The clockwise direction (left) is called spin up; the counterclockwise rotation (right) is referred to as spin down.
Eerily, one can concoct superpositions of up and down, just like the ghost states of Schrödinger’s cat. An electron can be 80 percent up and 20 percent down, and its future dynamics can be understood only if we assume that it evolves in this psychotic state. If you don’t believe these oddities of “quantum spin” neither should you believe the results of your MR scan. Or trust your mobile phone, computer, TV set, automobile, etc. Dislike quantum mechanics all you want, but modern technology is predicated on it.
But where exactly is Lilliput? Just how small is “very small”? It can mean different things in different contexts, such as in the diverse subjects of atomic and nuclear physics. The two fields involve quite different creatures: Atomic physics deals with electrons whizzing around the nucleus; nuclear physics, with the dark secret of the nucleus itself. It wasn’t until the 1930s that we first accessed the interior of the nucleus; the orbiting electrons, meanwhile, have been probed ever since the birth of chemistry.
The Boys first worked in atomic physics—it was their scientific nursery, where they honed their quantum-mechanics skills. We learn much about the electrons surrounding the nucleus from the light emitted when they transit between different quantum states: That’s how quantum mechanics, with its package of oddities, was first revealed. Franco Rasetti was an expert in measuring this “spectral light,” and Fermi was a leader in its mathematics. Segrè and Amaldi fawned upon these two, performing menial duties with enthusiasm and diligence. Ettore stood aside, his role, seemingly, to prove them all wrong.
Ettore’s first known scientific intervention occurred in 1928, when he was only twenty-two years old. It was a communication to the Italian Physical Society, in which he corrected the Thomas-Fermi model, a way of dealing with multiple electrons in an atom by replacing them with “electron clouds.” Fermi adamantly disagreed, which didn’t stop him from publishing the same work six years later in collaboration with Amaldi, giving no credit to Ettore.
In his “atomic physics” period, Ettore published half a dozen papers, all single-author apart from one. Of these, his best-known work is the discovery of how “spectral light” shifts when atoms are bathed with an oscillating magnetic field. The result, the Majorana-Brossel effect, nowadays carries his name, and the mathematical technique used to solve the problem is called the Majorana sphere (still much in use; for example, in the beautiful formalism devised by Roger Penrose to describe spinning particles). Modern MR scans are an offshoot of Ettore’s work.
But it was in nuclear physics that Ettore and, later, the Boys would leave their most indelible mark, beginning with Ettore’s prediction of the neutron. Given their later role as experts on the topic, it’s extraordinary that the Boys struggled to recognize the neutron’s existence until as late as 1933. They weren’t alone. At the time, almost without exception, scientists believed the nucleus to be made of protons and “nuclear” electrons. The idea was based on common sense. But common sense is seldom the path to truth.21

How were nuclei and atoms envisaged when Ettore disturbed the status quo? The hydrogen atom, for one, was easy to figure out. A hydrogen atom contains one nuclear proton orbited by one electron. The electron is much lighter than the proton—1,836 times lighter—so that most of the atomic mass is in the nucleus: The mass of the hydrogen atom is roughly the mass of the proton. Because the proton and the electron have the same charge with opposite signs (positive for the proton, negative for the electron), the atom is electrically neutral. Nothing about the hydrogen atom was controversial; the issue was how to account for heavier nuclei.
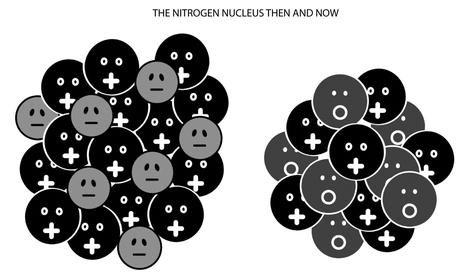
Take nitrogen, for example. A nitrogen nucleus weighs roughly fourteen times as much as a proton, but it has a (positive) charge only seven times larger. The nucleus is orbited by seven electrons (rendering the atom neutral), but let’s forget about those for the moment. Focusing on the nucleus, the question was how the nucleus could be fourteen times heavier than a proton but only have seven times its charge. The textbook answer was to employ fourteen protons and seven intranuclear electrons.22 The charge comes out right because 14-7 = 7 (seven electrons neutralizing seven protons); and the mass also works out because the mass of the electrons is negligible, and there are fourteen protons in the nucleus (see Figure 6.2).
Believing the nucleus to be made of protons and electrons was therefore far from stupid, particularly considering that the fad of postulating thousands of unnecessary particles—as in string theory—was still well in the future. Combine this with the fact that during beta decay the nucleus emits one electron (suggesting that this electron already lived inside the nucleus), and you’d have to be an outright lunatic to come up with any other model for the nucleus. Which is exactly what Ettore did.
Figure 6.2: The nitrogen nucleus as it was understood before and after the neutron entered the picture. Charge seven and mass fourteen can be explained either with fourteen protons and seven nuclear electrons, or with seven protons and seven neutrons.
Because in reality there was a need for the neutron, as should have been obvious since the 1920s. Much as the Boys resisted it, of all people they should have known better. No one more so than Franco Rasetti, who was sent abroad by Fermi in 1929 “to season,” and found himself at Caltech studying the properties of the nitrogen nucleus, just as in the example I chose. The Cardinal Vicar’s project was to measure the spin of this nucleus.
When you combine spins in groups, they add or subtract according to well-defined rules. Two spin half particles can only add up to a spin one (when both spins align in the same direction: ½ + ½) or spin zero (when the spins point in opposite directions: ½-½). Quantum mechanics tells you the probabilities of one pairing over the other. And similar addition laws rule larger conglomerates of quantum particles. Rasetti found that the nitrogen nucleus has spin one.
This utterly contradicted a nucleus made of fourteen protons and seven electrons, because such a nucleus would have an odd number (twenty-one) of particles. No matter how you pair and combine an odd number of spin half particles, you can only produce a half-integer collective spin: ½,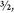 ,
,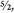 , etc. Yet the total spin of the nitrogen nucleus was one. A puzzling result—ignored by everyone and quickly forgotten.
, etc. Yet the total spin of the nitrogen nucleus was one. A puzzling result—ignored by everyone and quickly forgotten.
Except by Ettore. He must have been the first to understand that if the nitrogen nucleus contained protons and a new kind of spin half particle—the neutron—then Rasetti’s observation could be explained. In contrast to Amelio’s film, Ettore did talk openly to the Boys about a “neutral proton”: a particle with a mass similar to the proton, no electric charge, and spin half. He noticed that given its existence, the nitrogen nucleus could be made up of seven protons and seven neutrons, accounting for mass fourteen and charge seven. And then there would be an even number (fourteen) of spin half particles in the nucleus, so that their spins could add up to an integer number. Such as one, the observed value.
The clues were all there—at the Panisperna labs! And Niels Bohr, the father of quantum mechanics, had already remonstrated about “the remarkable passivity of intranuclear electrons,” which somehow lost even their spin as soon as they were inside the nucleus. Yet these clues went unheeded, and Ettore did in fact “see beyond,” as portrayed in Amelio’s film, when he predicted the neutron’s existence. But there was more to it than the nitrogen spin experiments: There was something else of crucial importance that may have scared him.
Rasetti’s discovery may have been important, but there was at the time another much more blatant conundrum in nuclear physics: No one understood why a nucleus made of protons and electrons, governed only by electricity, didn’t simply blow apart. The energy budget of a nucleus governed by electric attractions and repulsions favored its quick dismantling. Scientists envisioned the nucleus as a small molecule, which is indeed stable due to the collective electric attractions and repulsions of its components. But the nucleus is 100,000 times smaller than any molecule, and for something that small, electric repulsion wins in the overall budget. It just doesn’t scale, as a simple calculation (surely performed by Ettore) quickly shows. In order to explain why the nucleus was bound together, a new type of force, stronger than electricity, was needed, as well as a new, electrically neutral particle.
We find this prescient remark in Ettore’s notebooks when he was in his early twenties. He wasn’t being stubborn: He’d tried to set up a nuclear model using the conventional picture and had come up with nonsense. He wasn’t proposing a new particle and a new force gratuitously—he was seriously attempting to explain the mysterious stability of the nucleus. And he’d found that with his new particle and force, not only was the nucleus stable, but its binding energy was millions of times larger than that binding electrons in atoms. Did he gasp in horror upon recognizing the enormous strength of nuclear forces? We don’t know. No one listened to him on the few occasions he mentioned neutrons and strong forces to the Boys.
The picture changed dramatically when the Joliot-Curie husband and wife partnership accidentally produced the first free neutrons. They had no idea what they’d found but, as good experimentalists, gave an accurate account of what they’d seen. This new radiation could easily approach or even penetrate nuclei and produced such gigantic knocks on these nuclei that its particles had to be at least as massive as the proton. Everyone, including the Joliot-Curies, was baffled. What the hell was going on?
It’s telling that Ettore, upon hearing the news, chose to play the Inquisitor: “They haven’t understood anything! It’s probably the result of recoil protons produced from heavy neutral particles,” Ettore said, according to Amaldi. The Joliot-Curie particle was like a proton but had to be neutral: If it were positively charged, it would have to overcome an enormous repulsion barrier to penetrate the (positively charged) nucleus. For someone like Ettore who had worked with neutrons for several years, the Joliot-Curie results were therefore hardly surprising.
Meanwhile, Fermi and the Boys were utterly in the dark. Even Werner Heisenberg, who officially proposed the first theory of nuclear stability, couldn’t quite believe in the new particle. To illustrate the generalized mental block, I note that the great Werner understood the “neutron” as a “little atom” made of one proton and one electron. In effect, he simply repackaged the old nuclear model of protons and electrons, combining some of the electrons and protons into couples. Rasetti’s experiment clearly hadn’t sunk in. The neutron has spin half, whereas such couples would have spin one or zero. No wonder Ettore felt impatient.
Not only was Ettore sure of the neutron’s existence, he had also finished a theory of nuclear stability (and isotope instability) based on protons and neutrons, bound by a new type of force he dubbed “force of exchange.” This was the precursor of the strong force and later quantum chromodynamics (or QCD), and it was a most remarkable breakthrough. Ettore didn’t burn it, but he never published it, vehemently refusing to do so to everyone’s puzzlement. Although Fermi had yet to be convinced by the argument, he realized that Ettore’s theory might be right and what a coup it would be for Via Panisperna. He insisted that Ettore publish it—to no avail.
So upset was Fermi that he even suggested presenting the theory at an important congress in Paris, giving due credit to Ettore. Ettore agreed, but on condition that the theory be attributed to a certain professor of electronics in Rome, widely regarded as a moron, who was bound to attend the conference. Fermi didn’t have the guts for the prank. And so Ettore’s idea remained in the drawer.
A few months later, Dmitri Ivanenko in Russia recognized the new particle, and Heisenberg in Germany proposed much the same theory as Ettore. When Fermi expressed his disappointment, Ettore only laughed. He found the entire situation hilarious. And when Fermi suggested publishing at least a follow-up, Ettore—having refused to publish before Heisenberg—said, “Now Heisenberg has already done everything.” And he chortled away while the other Boys commiserated.
How can we ever understand Ettore’s bizarre behavior? Was he disturbed by the large nuclear binding energies he’d found; did he foresee their deadly potential? Or are there simpler reasons? For someone as driven by competition as Fermi, Ettore must have been impossible to comprehend. And the inner workings of such psychotic behavior can’t be trivial.
Writer Leonardo Sciascia23 offers a disconcerting explanation. He compares Ettore to Stendhal, who dreaded his precocity so strongly that he squandered as much time and talent as possible in a vain attempt to “delay” his genius. “Like Stendhal, Majorana tries to avoid accomplishing what he has to accomplish, what he can’t help accomplishing,” Sciascia writes in his book on Ettore. The Boys were keen in their pursuits, had unbeatable enthusiasm and stamina, but they were “seeking”; Ettore, in contrast, just “found.” But such miracles came with a price. Ettore must have sensed death and self-destruction in each of his revelations. They were so extreme that they became sinister, malignant. In this mental climate, throwing away Nobel Prize-worthy work might actually have been an act of self-preservation, Sciascia suggests. So “when Heisenberg’s theory is eventually acknowledged and publicized, not only does Majorana refrain from lamenting . . . but conceives for the German physicist a feeling of sincere admiration (based on self-esteem) and gratitude (based on dread). Heisenberg represents for him an unknown friend—someone who without knowing him, has saved him from disaster, enabled him to avoid a sacrifice.”
I like Sciascia’s affinity for poetic license. How else could one ever explain utter madness?
Back at Via Etnea in Catania, I’d been told I should meet Ettore Majorana, because he now kept the private documents found in Dorina’s bedside safe. And so I go to Rome, where Ettore’s scientific dramas originally unfolded. The building at Via Panisperna is still there, sadly no longer housing the Physics Department. I have to go instead to the new University City, not far off. That’s where Ettore has his office.





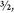 ,
,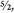 , etc. Yet the total spin of the nitrogen nucleus was one. A puzzling result—ignored by everyone and quickly forgotten.
, etc. Yet the total spin of the nitrogen nucleus was one. A puzzling result—ignored by everyone and quickly forgotten.
